A recent study in the Rabinovitch Lab has shown that an anti-oxidant compound, called SS-31, effectively protects against heart failure in mice, demonstrating a potential therapeutic value for human heart diseases. The study, Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides, was published in the September 2013 issue of Circulation: Heart Failure.
Heart failure is the most common reason for hospitalization of the elderly, and the number one cause of death in individuals over the age of 65. With advancing age, the heart progressively becomes weaker and dilated, eventually losing the ability to pump enough blood to sustain the body. Within the heart’s beating muscle cells (called cardiomyocytes), rising levels of damaging oxidants and declining efficiency in cellular energy production may underlie or exacerbate this decline in heart function.
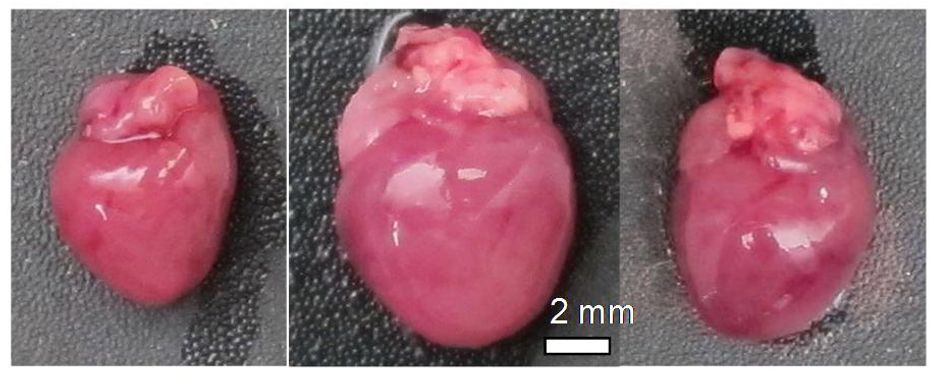
Researchers in the Rabinovitch Lab hypothesized that by administering SS-31 to mice during heart failure, they could lower levels of these damaging cellular oxidants and protect against the development of heart failure and dysfunction. The team utilized a surgical procedure, known as trans-aortic constriction, to induce heart failure in mice that mimics the primary features of heart failure in humans: including cardiac hypertrophy (enlarged heart), systolic dysfunction (failure of the pump function of the heart), diastolic dysfunction (decline in performance of one or both ventricles during cardiac cycle phase in which the heart relaxes and fills with incoming blood), and scarring. While the mice that underwent surgery exhibited all of these features of heart failure, the group that received SS-31 treatment following surgery had hearts that looked and functioned more like a healthy heart.
Given that most anti-oxidants have failed to show therapeutic value in the past, the researchers speculated that SS-31 was able to protect the mouse hearts because of a unique ability to target the power generators of the cell, known as mitochondria. To examine these cellular changes the team used “proteomic” methods to compare changes in about a thousand proteins from all groups of mice. As expected, heart failure significantly reduced the proteins involved in mitochondria as well as energy production. Treatment with SS-31 had the effect of maintaining these proteins at the levels typical of young healthy mice, even though the surgical procedure without SS-31 caused radical changes in the proteomes of mice as it produced heart failure.
These findings give hope that SS-31 may eventually be an effective treatment for heart failure in humans, and suggest that mitochondrial targeted therapeutics may provide a powerful tool for fighting heart disease.